Safety and performance
Hydrolyzed collagen is absolutely non cytotoxic. Instead, in vitro experiments show it increases all parameters measuring cell vitality (1), (4). No immunogenic effects have ever been observed in vivo (5). It has been shown to be effective on degenerative articular disorders both in pre-clinical and clinical studies published in international, peer-reviewed journals (3), (4), (6), (7). Such studies include double-blind randomized clinical trials on patients affected by knee osteoarthrosis (8), (9).
CHondroGrid® is manufactured starting from pharma-grade highly purified bovine collagen. The extraction process applied to purify the CHondroGrid® bovine collagen has been certified safe by the European Directorate for the Quality of Medicines (EDQM) as far as the TSE (Transmissible Spongiform Encephalopathies) risk is concerned. During its production, Bioteck further checks the raw material for the absence of prions using biomolecular assays, such as Western Blot Test and HPLC chromatography. Safety and performance assessments performed on the CHondroGrid® device have been validated by all the Member States of the European Community.
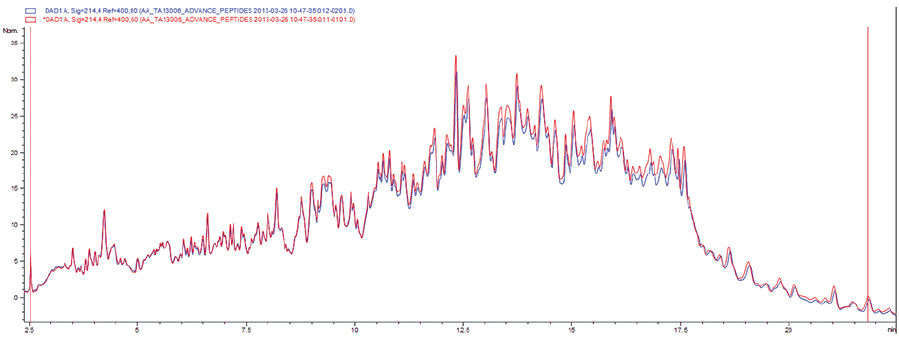
Manufacturer performs strict microbiological tests on each product batch and monitors, along the entire production line, all the environments where the device is manufactured and packaged. After packaging, the product is sterilized by beta irradiation at a 25 kGy dose. The irradiation preserves all the qualitative and quantitative features of the peptide profile, causing no decay of the device performance. HPLC data recorded before and after sterilization show that the chromatographic peaks fully overlap, as shown in the plot that follows.